
This longitudinal study is made up of multiple waves of data collection examining the neurodevelopmental progression of self-regulatory behavior and emotion competence, starting at the very beginning of life by evaluating in utero brain functional connectivity.
We focus on the child and mother to ultimately explore the healthy neurodevelopment of children and fetuses; bases for cognitive control, emotion regulation, and the development of psychopathology; the connectivity of functional networks in the developing brain; and other factors that influence variation in pediatric neurobehavioral development.
We aim to link measures of child health back to prenatal factors, such as fetal brain connectivity and maternal psychological stress.
Perinatal Imaging of Neural Connectivity
pregnancy & infancy

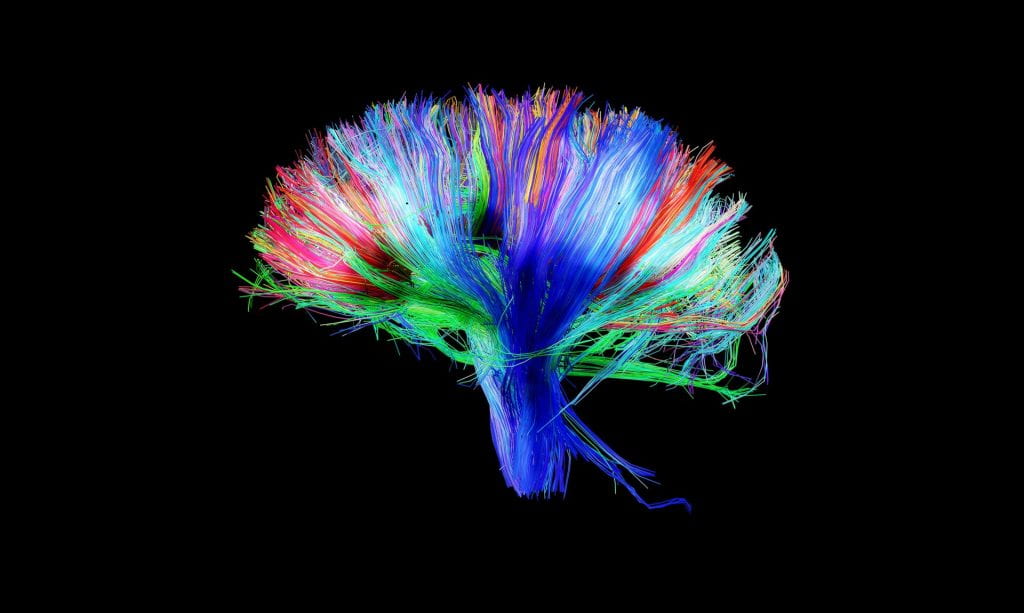
Following the recruitment of expecting mothers, the first wave of data acquisition, which was led by Dr. Moriah Thomason, involved functional (resting-state) and structural (diffusion tensor) neuroimaging methods of the human fetus to model healthy and atypical brain development in utero. We aimed to establish the sequence and patterning of fetal functional neural networks.
Prior to entering the MRI scanner, the mother completed a number of questionnaires to assess aspects of her emotional and physical state, including her mental and physical health, socioeconomic status, and attachment style.
In addition to these tasks, maternal salivary cortisol samples and maternal blood samples were collected.
Following this visit, we then further mapped the emergence of the human connectome by inviting mothers back with their child after giving birth. Here, infants were studied during natural sleep using resting-state, diffusion tensor (DTI), and functional MRI tasks (passive processing of emotional non-words, exposure to maternal smell). Mothers completed various questionnaires, and infant salivary cortisol samples were collected at this visit.
Early Life Risk, Resilience, and Behavioral Outcomes
age 7 months, 36 months

Mom and child were invited back to the lab when the child reached seven months old, and again once the child reached 36 months old.
During the seven-month visit, the mother and child engaged in the Still-Face Paradigm to assess child socioemotional regulation.
During the 36-month visit, the mother-child dyad completed interactive tasks. Dr. Marjorie Beeghly’s team codes these tasks using an adaptation of the Parent-Child Interaction System (PARCHISY).
During both visits, the Bayley Scales of Infant Development were completed to assess child developmental functioning. At both visits, mothers completed questionnaires that include measures of infant behavior, parenting experiences, health, and life circumstances.
Child cortisol, height, weight and blood spots were also collected.
Early Childhood Neurodevelopmental Health Outcomes
age 5 years

We are in the process of following up with children when they reach 5 years of age.
During an initial home visit, we assess the sleep environment, provide an ActiGraph wristband for the child to wear for the measurement of child sleep and physical activity, and deliver diurnal cortisol sample collection materials for the mother to collect from her child. We also interview the mother during this visit to assess her child’s traumatic experiences.
The mother and child then participate in another lab visit. Here, a number of measures are completed including child height, weight, and assessments of school readiness and emotion regulation. Mother-child interaction is also recorded. The child also completes several computer tasks to measure executive functioning and additional tasks to measure emotion regulation with electroencephalography (EEG).
The Tooth Fairy Project
age 3 — 11 years
We collect naturally shed baby teeth from our longitudinal cohort to assess toxicant and micronutrient exposures and inflammatory markers in collaboration with Dr. Christine Austin and her research team at Mt. Sinai.
Follow-up Assessment of Inflammation and Neurobehavioral Development
age 9 years
Our team recently started another follow-up assessment at age 9-10 years. This wave aims to identify whether inflammation in the human prenatal period exerts influence over the developing fetal neural connectome and subsequently increases risk for problem behaviors and disorders in childhood.
This project involves the pairing of new advances in resting-state functional connectivity MRI with innovations in tooth-biomarker assays using the deciduous tooth samples collected throughout the Tooth Fairy Project. Data collection involves the following:
- An initial home visit in which families are given an ActiGraph wristband to measure child sleep and physical activity as well as a diurnal cortisol collection kit. A child blood sample is also collected. Children additionally complete various neurobehavioral assessments (executive functioning, attention, memory, language development) using tests included in the NIH Toolbox, as well as a matrix reasoning task to assess neurocognition.
- A lab visit that includes a child MRI using the same scanner that was used prenatally. Behavioral observation includes a conflict discussion task between the mother and child.
Throughout both visits, mothers complete a number of questionnaires assessing factors such as her physical health, family cohesion, neighborhood, child’s behavior, maternal depression, maternal anxiety, parenting, maternal perceived stress, and perceived discrimination. Children also complete questionnaires, which include measures of child depression, anxiety, trauma, resilience, behavioral approach, and physical health.
This wave of data collection is intended to be partially harmonized with the NIH Adolescent Brain Cognitive Development (ABCD) Study protocol.
