Contents
Neuroinflammation/Metabolism/Aging
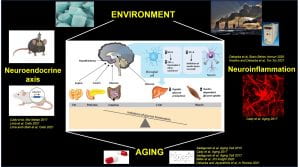
Our research program focuses on the hypothalamic regulation of metabolism in states of environmental stress, obesity, and aging. The hypothalamus integrates information from the liver, muscle, fat, and other organs, and orchestrates whole-body metabolic homeostasis. We study signaling pathways operating in hypothalamic neurons and glia cells (astrocytes and microglia), which are highly relevant to the control of systemic metabolism and the age-associated changes in the glia-neuron interactions.
Our lab employs a multi-disciplinary approach to manipulate brain neurocircuits and nutrient-sensing pathways using cutting-edge molecular, genetics, and metabolic assessments in rodents.
The role of hypothalamic neuroinflammation in the aging brain
Aging is characterized by a progressive increase in neuroinflammation, which contributes to cognitive impairment, associated with neurodegenerative diseases. Aging associates with activation of microglia (brain resident macrophages) and astrocytes, and pathological production of proinflammatory cytokines. This leads to aberrant neuronal signaling and deterioration of the central nervous system (CNS), all contributing to accelerated cognitive impairment. The detailed mechanisms controlling neuroinflammation are still unknown. The interplay between aging, metabolic changes, and systemic/central inflammation suggests that therapeutics that target metabolism and age-associated inflammation may promote healthy brain aging and can possibly be used as a treatment for neurodegenerative diseases.
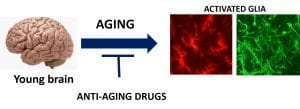
We are focusing on the role of neuroinflammatory signals in the aging brain, with a major focus on age-dependent changes in hypothalamic inflammation and metabolic regulation. Our recent studies involve the assessment of anti-aging drugs on sex-specific neuroinflammatory responses in the hypothalamus and cognitive function.
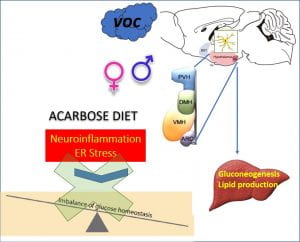
Exposure to air pollution is a trigger for hypothalamic inflammation and metabolic disease
The goals of this project are to define the mechanisms by which air pollution affects metabolic imbalance, identifying molecular mediators with specific emphasis on hypothalamic neuroinflammatory pathways that may ultimately have implications for potential interventions.
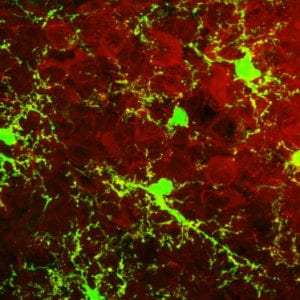
Volatile organic compounds (VOC), are major components of air pollution, contributing to both outdoor and indoor pollution. VOCs can enter the CNS, through direct transport or via systemic inflammation upon initial recruitment of immune cells in the lung tissue. Emerging evidence suggests that microglia and astrocytes play critical roles in the regulation of immune and inflammatory responses particles or reactive gases of urban air pollution in the CNS. Microglia, the major cell type responsible for CNS immune response, are exquisitely sensitive to stressors and are activated by inhaled components of urban air pollution through both direct and indirect pathways.
Our new studies indicate that neuroinflammatory signals of the hypothalamic glia cells are rapidly activated in response to VOC exposure and contribute to the whole-body metabolic imbalance.
Using novel genetically engineered mice models we are currently assessing the role of microglia-specific inflammatory pathway and ER stress response on the whole-body metabolism in response to VOC exposure.
https://www.sciencedirect.com/science/article/abs/pii/S0889159120303457
The effect of perinatal exposure to VOC on hypothalamic development, neuroinflammation, and whole-body metabolism in the offspring
Microglia colonize the fetal brain during the latter half of gestation. During this time, they play a critical role in the cross-talk between the nervous and immune systems as well as in several developmental processes. Activation of the immune system during pregnancy or early life may contribute to the etiology of metabolic disorders.
Our new study demonstrates that gestational exposure to VOC (using benzene as a model VOC) induces deleterious effects on energy homeostasis and insulin resistance in adult offspring.
https://academic.oup.com/toxsci/article-abstract/180/2/252/6121171?redirectedFrom=fulltext
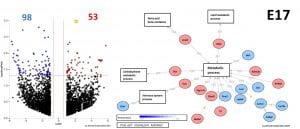
Using innovative technical approaches including novel mice models, and hypothalamic transcriptional profiling we now assess the impact of perinatal VOC exposure on offspring hypothalamic development, and inflammatory response in the hypothalamus, as a potential risk factor for late-life metabolic disorders.
Hypothalamic neuroendocrine axis in aging and metabolism
The brain detects alterations in energy balance and glucose levels through autonomic and neuroendocrine controls. Hypothalamic neuroendocrine circuits coordinating growth hormone (GH) secretion operate within complex physiological settings, regulating proper responses to various stressors. We are utilizing unique mouse models to analyze a novel population of hypothalamic neurons that are critical for the feedback inhibition of GH production, in controlling systemic glucose homeostasis and regulate aging processes.
https://www.sciencedirect.com/science/article/pii/S2212877817301138
Using designer receptors exclusively activated by designed drugs (DREADDs; expressed as DREADD-mCherry fusion proteins) technique to control the GHR+ neuronal activity, we demonstrated that activation of GHG expressing neurons in the hypothalamus promotes whole-body glucose utilization and significantly improves glucose tolerance. Furthermore, using state-of-the-art techniques to determine in vivo insulin sensitivity (hyperinsulinemic-euglycemic clamp), we have identified GHR-expressing neuronal population in the hypothalamic arcuate nucleus (ARC) as a major regulator of glycolysis and muscle insulin sensitivity.
https://www.mdpi.com/2073-4409/10/5/1093
https://www.mdpi.com/2073-4409/10/4/891

We are currently exploring molecular mechanisms in the hypothalamus that trigger an age-associated shift in energy homeostasis induced by the GH axis, which underlie the metabolic imbalance in aging.